Machine Learning extension to Hyperdynamics
Hyperdynamics is an Accelerated Molecular Dynamics method used to "speed up" simulations, while still enabling direct and accurate calculations of dynamic properies. This method is proven to be useful in "hard" systems, like metal materials, but needs significant adaptations to be useful for "soft" systems like proteins. In collaboration with Art Voter at Los Alamos National Laboratory, I used clustering and classification techniques to begin development of Hyperdynamics for proteins.
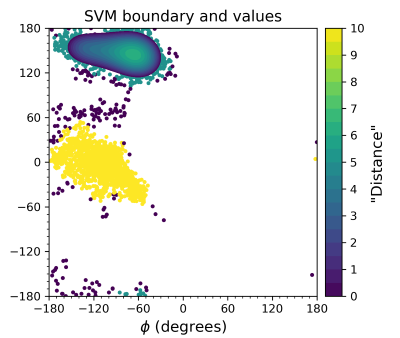
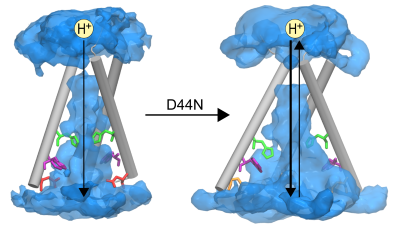
Proton Transport in Designed Protein Channels
Selective proton transport through proteins is essential for forming and utilizing proton gradients in cells. Protons are conducted along hydrogen-bonded “wires” of water molecules and polar sidechains, which, somewhat surprisingly, are often interrupted by dry apolar stretches in the conduction pathways inferred from static protein structures. Here, we used molecular dynamic simulations to aid the design of minimal transmembrane channels capable of conducting protons with rates similar to viral proton channels, informing future engineering of proton-conducting materials.
Proton-Induced Conformational and Hydration Dynamics in the Influenza A M2 Channel
The influenza A M2 proton channel is responsible for the acidification of the viral interior after the virus enters a cell--a critical step in the infection process. Here, we performed exploratory data analysis with data from over 100 simulations to deduce the structural and dynamic changes that accompany proton flux and enable channel activation. We found that the protein structure shifts based on the proton position, and demonstrated how the hydrated excess proton stronly influences the water network throughout the channel.
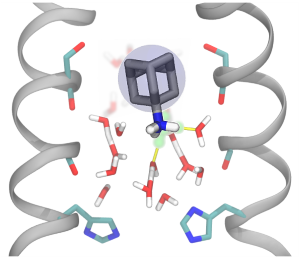
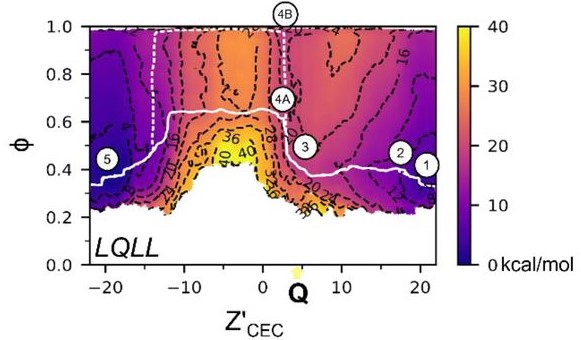
M2 Inhibitor Binding Understood through Mechanisms of Excess Proton Stabilization and Channel Dynamics
The influenza A M2 proton channel is drug target, and two of the six available antivirals target M2, but drug-resistance has rendered them ineffective. To help aid new drug design efforts, we investigated how the adamantane drugs inhibit M2 and found several key characteristics that make these inhibitors effective. In particular, we compare drug binding with proton stabilization in the channel to show how these drugs take advantage of the channel's natural ability to stabilize an excess charge. This work gives a more dynamic picture of drug inhibition, with critical implications for future drug design.
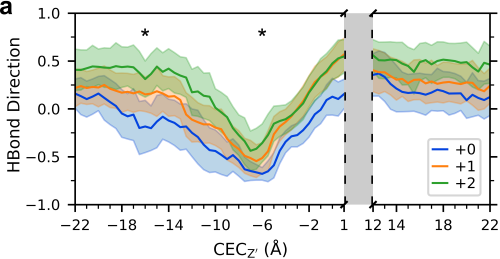
Multiscale Simulation of an Influenza A M2 Channel Mutant Reveals Key Features of Its Markedly Different Proton Transport Behavior
In this work, we use extensive Multiscale Reactive Molecular Dynamics (MS-RMD) and Quantum Mechanics/Molecular Mechanics (QM/MM) simulations to model proton movement through a prevalent mutant of the influenza A M2 channel. This mutant exhibits much higher conductance compared to wildtype M2, and an altered pH dependence. Using statistical mechanics to calculate the free energy of proton transport, we investigate how this mutation alters the proton transport mechanism and show the key role of water.